Our primer article “Retinal ganglion cells” has been published in Current Biology.
Akihiro Matsumoto, Keisuke Yonehara
Current Biology(2026)
https://doi.org/10.1016/j.cub.2025.12.014
Our primer article “Retinal ganglion cells” has been published in Current Biology.
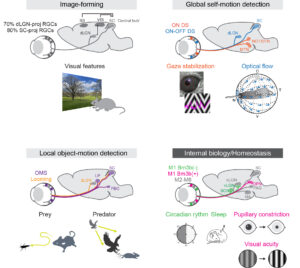
This primer provides an overview of advances in research over the past 20 years aimed at identifying different types of retinal ganglion cells.
Decoupling of visual feature selectivity in the retinocollicular pathway
Ole S. Schwartz*, Akihiro Matsumoto*, Haruka Yamamoto, and Keisuke Yonehara
Current Biology(2025)*equally contributed.
https://doi.org/10.1016/j.cub.2025.11.050
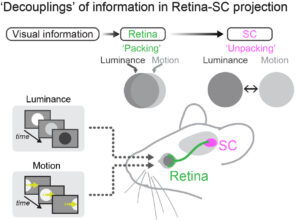
We discovered that while luminance responses can predict motion responses in retinal ganglion cells, this prediction does not hold in the superior colliculus. This feature selectivity decoupling reduces functional cluster separability in collicular neurons. Our work revisited the definition of functional cell types.
Functionally distinct GABAergic amacrine cell types regulate spatiotemporal encoding in the mouse retina.

Matsumoto A, Morris J, Looger LL, Yonehara K (2025) Nature Neuroscience, doi: 10.1038/s41593-025-01935-0.
Using two-photon GABA imaging, we classified amacrine cells into more than 40 functional types in the mouse retina. Molecular clustering was done in collaboration with the team of Dr. Looger of UCSD/HHMI. The iGABASnFR2 sensor was developed by Janelia GENIE project.
Direction selectivity in retinal bipolar cell axon terminals.
Matsumoto A, Agbariah W, Nolte SS, Andrawos R, Levi H, Sabbah S, Yonehara K (2021) Neuron, 109: 2928-2942.e8.
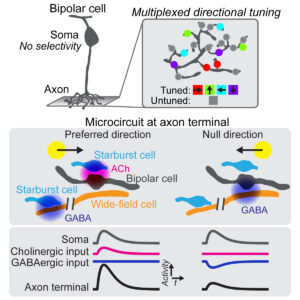
Using two-photon glutamate imaging, we found that visual motion is already computed at the axon terminals of bipolar cells in the retina. The 3D electron microscopy analysis was done in collaboration with Dr. Sabbah of Hebrew University.
Rapid multi-directed cholinergic transmission in the central nervous system.
Sethuramanujam S§, Matsumoto A§, deRosenroll G, Murphy-Baum B, McIntosh JM, Jing M, Li Y, Berson D, Yonehara K*, Awatramani GB* (2021) Nat Commun 12: 1374. § equally contributed. *shared-corresponding authors.
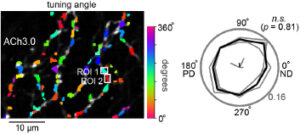
Using two-photon acetylcholine imaging, we found that acetylcholine can transmit information regardless of the presence or absence of synaptic structures as long as 1-2 microns from the release site. The research was conducted in collaboration with Dr. Awatramani of the University of Victoria.
Binocular integration of retinal motion information underlies optic flow processing by the cortex.
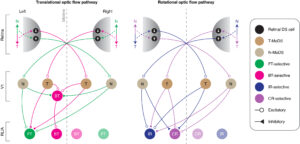
Rasmussen NR*, Matsumoto A*, Arvin S, Yonehara K (2021)
Curr Biol 31: 1165-1174. *equally contributed.
In vivo two-photon calcium imaging showed that visual motion signals from both eyes are specifically combined in each area of the visual cortex to form translational and rotational optic flow responses.
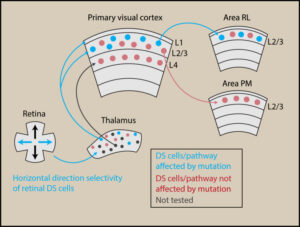
Contributions of Retinal Direction Selectivity to Central Visual Processing
Rasmussen R, Yonehara K (2020) Curr Biol 30: PR897-R903. [PDF]
Review Article. Summary of the contribution of retinal directional selectivity to information processing in visual centers such as the superior colliculus and visual cortex.
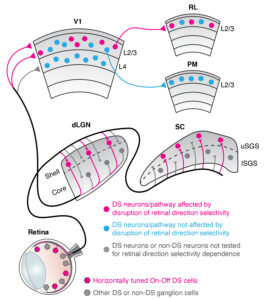
A segregated cortical stream for retinal direction selectivity.
Rasmussen R*, Matsumoto A*, Dahlstrup Sietam M, Yonehara K (2020) Nat Commun 11: 831. *equally contributed [PDF]
In vivo two-photon calcium imaging showed that direction-selective signals in the retina reach the second/third layer of the primary visual cortex directly via the lateral geniculate nucleus, and then are selectively sent to the Rostrolateral (RL) area of the higher visual cortex. This finding challenged a traditional view that cortical direction selectivity is computed within the cortex.
Spatiotemporally Asymmetric Excitation Supports Mammalian Retinal Motion Sensitivity.
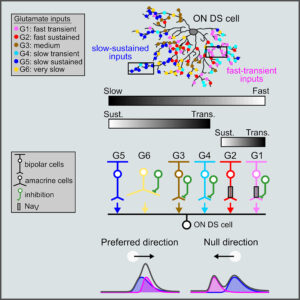
Matsumoto A, Briggman KL, Yonehara K (2019)
Curr Biol 29: 3277-3288. [PDF]
We found a spatiotemporal coupling motif between ON-type direction-selective cells and their excitatory input cells, bipolar cells, in the retina, which are visual motion detectors in optokinetic nystagmus. The 3D electron microscopy analysis was done in collaboration with Dr. Brigman at the Bonn-Caesar Institute. This is the first memorable original work from Yonehara Laboratory, Aarhus University.
Congenital Nystagmus Gene FRMD7 Is Necessary for Establishing a Neuronal Circuit Asymmetry for Direction Selectivity.
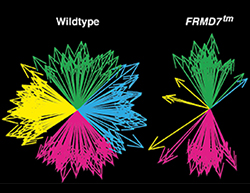
Yonehara K*, Fiscella M*, Drinnenberg A*, Esposti F, Trenholm S, Krol J, Franke F, Scherf BG, Kusnyerik A, Müller J, Szabo A, Jüttner J, Cordoba F, Reddy AP, Németh J, Nagy ZZ, Munier F, Hierlemann A, Roska B (2016)
Neuron 89: 177-193.*equally contributed. Best of Neuron 2016[PDF]
Congenital nystagmus, characterized by the absence of horizontal optokinetic reflexes, is a frequent visual disorder. We identified FRMD7 as a key molecule for asymmetric neural connections and showed that congenital nystagmus is caused by disruption of retinal directional selectivity. The paper was selected by Neuron as one of the best papers of 2016. Drs. Marla Feller and Josh Saines praised the work as the best contribution to the field.
