Our ultimate goal in the Multiscale Sensory Structures Laboratory is to understand how brain function is created based on mechanisms at lower levels of hierarchy, including genes, molecules, cell types, circuits, and neural computation. Such hierarchical longitudinal studies will allow us to identify the cell types and circuits responsible for neurological diseases that may be subject to genetic repair, and furthermore, to identify genomic differences that are responsible for species-specific brain functions. The knowledge gained from these species comparisons will be particularly useful in understanding human systems and diseases using mouse models. Directionally selective neural circuits in the retina are ideal models for studying neural circuit computation at various levels of hierarchy, including genes, synapses, circuits, and behavioral control.
1. Molecular and activity-dependent mechanisms of synaptic specificity emergence
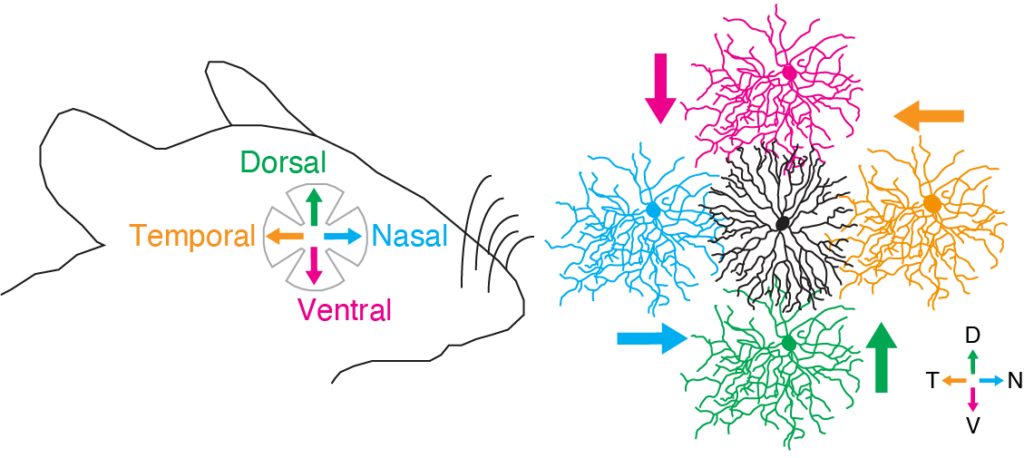
The animal body is formed in an orderly fashion along the body axis by a strict genetic program. Neural connections also have specificity along the body axis, and in particular spatial asymmetry of connections is a fundamental component of neuronal processing. Direction-selective (DS) cells in the retina have the property of responding selectively to visual motion direction and are involved in the detection of optic flow and optokinetic eye movements (Yonehara et al., PLoS One 2009). Starburst cell processes in the retina make an angle-dependent inhibitory coupling of processes to four types of DS cells (each with selectivity for dorsal, ventral, nasal, and ear directions), and this asymmetric inhibitory coupling is one of the key mechanisms underlying directional selectivity in the retina. This asymmetric coupling develops before eye-opening at 1 to 2 weeks of age (Yonehara et al., Nature 2011).
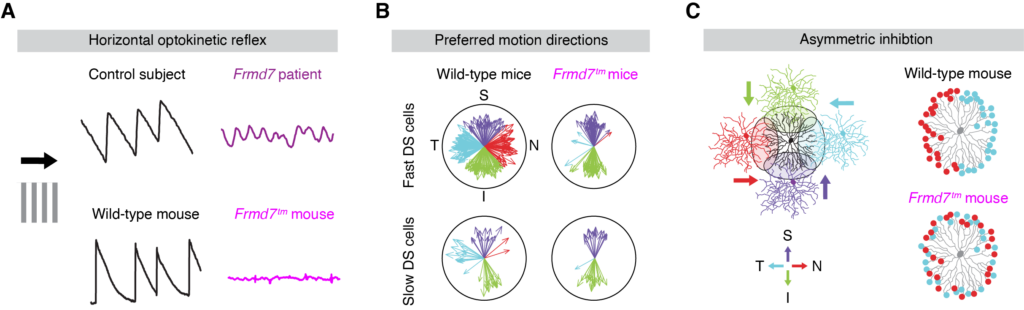
Previously, we found that Frmd7, a known causative gene of congenital nystagmus, is essential for the development of horizontally asymmetric inhibitory connections (Yonehara et al., Neuron 2016). In addition, we have identified several other important plasma membrane proteins and transcription factors (unpublished). Our future goal is to use these molecules as a starting point to identify completely new inter-synaptic and intracellular signaling pathways involved in the development of asymmetric connections in mammalian central nervous system neuronal circuits. To achieve these goals, we will combine a variety of techniques, including large-scale mouse genetics, biochemistry, cell biology, transcriptomics, proteomics, electrophysiology, and two-photon functional imaging, using the NIG's excellent animal breeding facilities.
2. Neural circuit mechanisms of visual information processing in the retina
We have previously identified the neural circuit mechanisms important for the processing of visual motion information in the mammalian retina. First, we performed two-photon glutamate imaging from the retina to identify space-time wiring from excitatory interneurons, bipolar cells, to DS cells and showed that this excitatory circuit motif underlies DS cell velocity tuning (Matsumoto et al. Curr Biol 2019). Next, we performed two-photon acetylcholine imaging from dendrites of DS cells and showed that the balance of excitatory/inhibitory inputs at the level of dendritic microsegments is important for direction selectivity calculations (Sethuramanujam et al, Nat Commun 2021). Subsequently, we made the unexpected discovery that direction selectivity in the retina is already computed at the axon terminals of bipolar cells (Matsumoto et al. Neuron 2021). Since axons are often thought of as simple conduits for transmitting signals, it was an unexpected finding that tuning to sensory stimuli occurs first at axon terminals. More recently, two-photon GABA imaging from the inner plexiform layer of the mouse retina revealed the existence of more than 40 functional GABAergic amacrine cell types that, thanks to their cell type-specific spatiotemporal dynamics, enable a variety of behaviorally meaningful visual feature extractions (Matsumoto et al. Nature Neuroscience 2025). Currently, we are conducting research to clarify how the diverse visual feature extractions of the retina dynamically change during the lifetime from birth to death.
To do this, we combine two two-photon imaging systems for explanted retinas in the lab, genetically modified animals labeled with cell types, patch clamp recordings, and data analysis with the aid of machine learning. Importantly, we have established international collaboration with groups in the US and China for receiving unpublished neurotransmitter sensors to conduct experiments as quickly as possible.
3. Sensory information processing and behavioral control in the superior colliculus and cerebral cortex
The mouse superior colliculus is an ideal model system for understanding how visual input from the retina is translated into innate vision-dependent behaviors. Recently, we found that the response of superior colliculus cells to light/dark and motion is statistically uncorrelated, suggesting that this is the fundamental principle of information conversion from the retina to the superior colliculus (Schwartz et al., bioRxiv 2023). We now plan to elucidate the cell types and circuit function of the superior colliculus by in vivo two-photon imaging under head-fixed conditions, imaging with a head-mounted microendoscope under free-moving conditions, transsynaptic labeling, and behavioral analysis.
We have found that signals from retinal ON-OFF DS cells selectively reach the rostrolateral (RL) region of the higher visual cortex (Rasmussen and Matsumoto et al., Nat Commun 2020), and that a combination of signals from retinal DS cells from both eyes in this region can form a response to rotational visual flow (Rasmussen and Matsumoto et al., Curr Biol 2021). We intend to continue our research on the circuit mechanisms of motor information processing in RL and their contribution to behavior.
In addition, as a new initiative, we have started a new project aimed at understanding the genetic mechanisms of environmental adaptation in visual neural circuits. We are comparing various visually dependent behaviors (e.g. prey hunting and escape defense behaviors) in various substrains of wild mice from around the world (known as Mishima battery) by behavior tracking using DeepLabCut in collaboration with Dr. Tsuyoshi Koide at the National Institute of Genetics.
To perform these studies, we use two in vivo two-photon imaging systems in the lab, one MINI2P (Thorlabs) in the lab that can be attached to the mouse's head and allows imaging under free-moving conditions, one Inscopix 1P miniscope in the lab, cell type-labeled genetically modified animals, combined with quantitative analysis of behavior using DeepLabCut.
4. Integration of sensory information in the medulla oblongata and regulation of systemic homeostasis
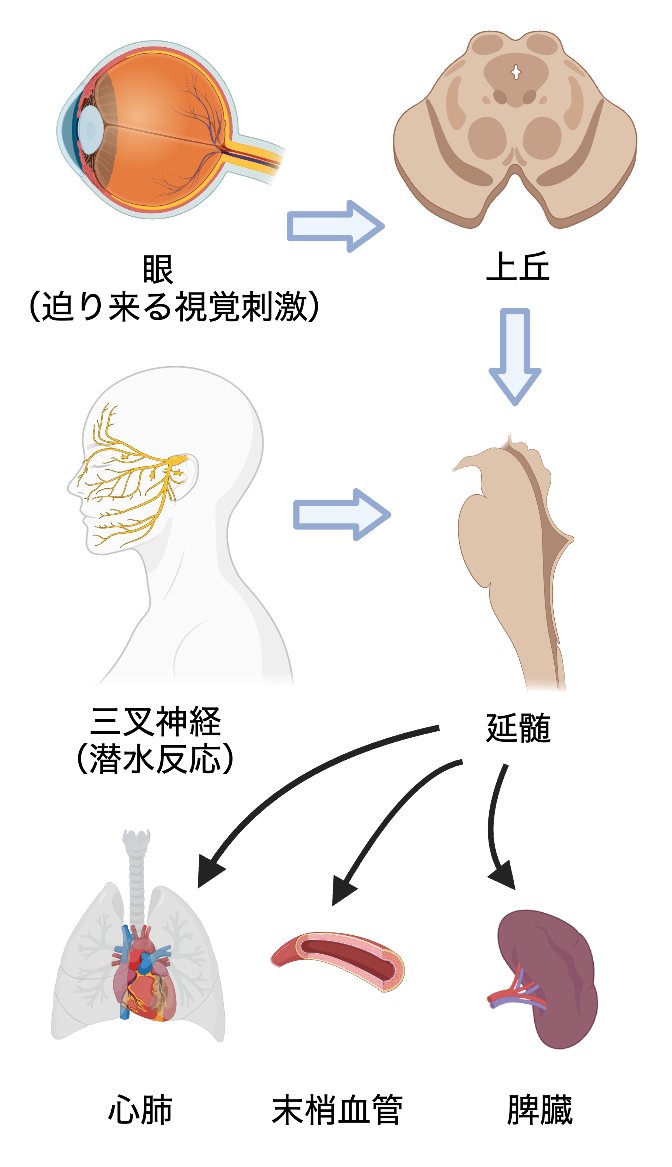
Life-threatening stress stimuli received by the sensory organs are integrated in the medulla oblongata and immediately trigger autonomic responses to maintain vital functions; however, much of the circuit infrastructure remains unknown. We are focusing on the visual threat response and the diving response to clarify the circuitry underlying the activation of the sympathetic nervous system by information integrated in the superior colliculus and the adaptive response involving the trigeminal nerve to hypoxic conditions by detecting diving into water, respectively. This research will reveal the influence of sensory information processing on the maintenance of vitality.
5. RNA-based strategies for treating genetic diseases
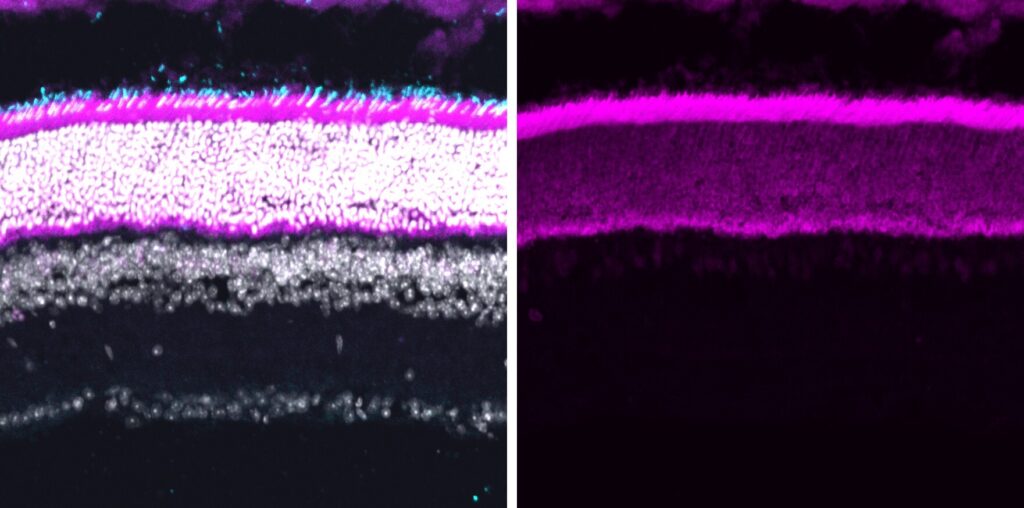
About 20% of genetic diseases are caused by nonsense mutation. By synthesizing artificial suppressor-tRNAs or snoRNAs, we aim to develop an efficient readthrough strategy that can be used for treating genetic diseases with nonsense mutation. We also develop animal models to use as an in vivo readthrough assay system, which will facilitate high-throughput screening of RNA variants. Our target diseases include blindness, muscular dystrophy, heart failure, and special types of cancer.